|
L. Kunze, S.-Y Tseng., R. Schweins, T. Sottmann, H. Frey, Langmuir 2019, 35, 15, 5221-5231. |
Carbon dioxide (CO2) is a renewable carbon source that is easily available in high purity and is utilized as a co-monomer in the direct ring-opening polymerization of epoxides to obtain aliphatic polycarbonates. In this work, degradable aliphatic polycarbonate diblock copolymers (mPEG-b-PBC) are synthesized via catalytic copolymerization of CO2 and 1,2-butylene oxide, starting from monomethoxy poly(ethylene glycol) (mPEG) as a chain transfer reagent. The polymerization proceeds at low temperatures and high CO2 pressure, utilizing the established binary catalytic system (R,R)-Co(salen)Cl/[PPN]Cl. Adding small amounts of the synthesized mPEG-b-PBC polymers to different microemulsion systems, we found that the polymers were able to strongly increase the efficiency of medium-chain surfactants to solubilize polar oils. |
|
M. Scharfenberg, J. Hilf, H. Frey, Adv. Funct. Mater. 2018, 28, 1704302. |
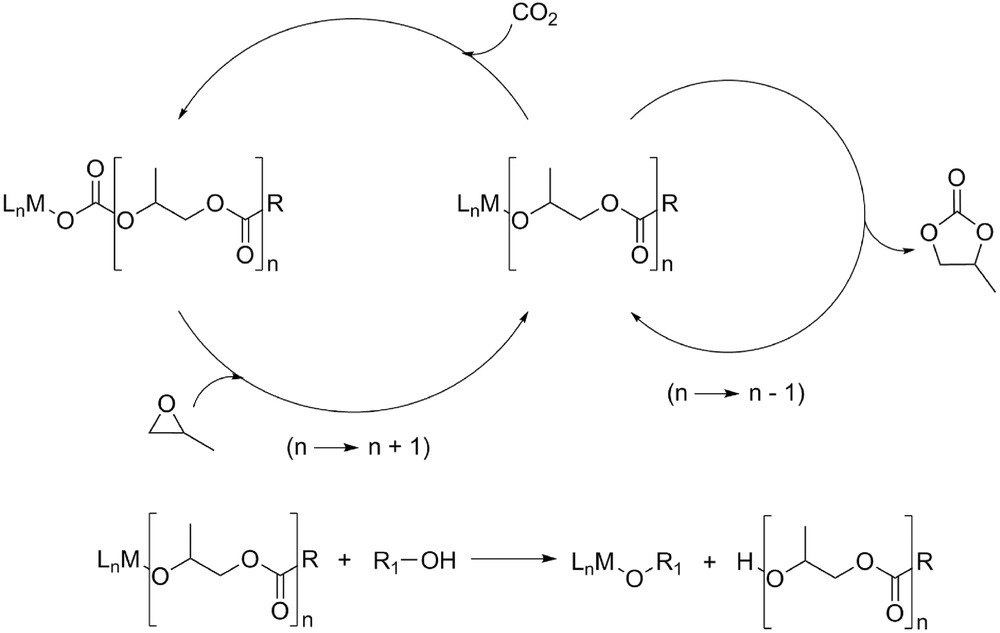
Aliphatic polycarbonates synthesized from carbon dioxide (CO2) and epoxides are resource‐saving, highly biocompatible and biodegradable polymers. Since the discovery of the copolymerization of epoxides and CO2 in 1969 by Inoue et al., this has become an important and useful technology for the large‐scale utilization of CO2 in chemical synthesis, employing mainly propylene oxide, and cyclohexene oxide (CHO). Only in recent years, functionalized polycarbonates have become an emerging topic with a broad scope of potential applications. This review summarizes synthetic routes and properties of numerous functionalized polycarbonates synthesized from CO2 and functional epoxide monomers. |