|
M. Scharfenberg, S. Hofmann, J. Preis, J. Hilf, H. Frey, Macromolecules 2017, 50, 6088–6097. |
Hyperbranched, multifunctional polycarbonate polyols based on CO2, cyclohexene oxide (CHO), and the “inimer” (initiator–monomer) (4-hydroxymethyl)-cyclohexene oxide (HCHO) were prepared in one-pot syntheses. The related linear poly(hydroxymethyl cyclohexene carbonate) structures based on protected HCHO and postpolymerization deprotection were also synthesized as model compounds. The content of hydroxyl functionalities was adjustable for both linear and hyperbranched terpolymer systems. All CO2/epoxide polymerizations were catalyzed by the (R,R)-(salcy)-Co(III)Cl complex. The hyperbranched polyols were also studied regarding their successful reaction with phenyl isocyanate to convert the free hydroxyl groups into urethanes. |
|
K. Wagener, M. Worm, S. Pektor, M. Schinnerer, Biomacromolecules, 2018, 19, 2506. |
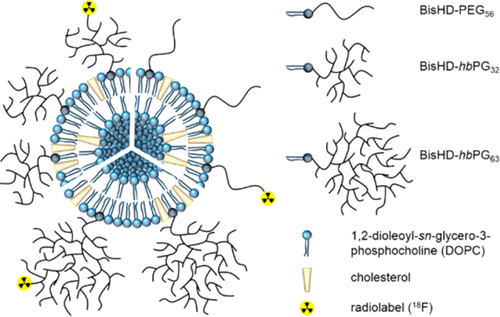
Multifunctional and highly biocompatible polyether structures play a key role in shielding liposomes from degradation in the bloodstream, providing also multiple functional groups for further attachment of targeting moieties. In this work hyperbranched polyglycerol (hbPG) bearing lipids with long alkyl chain anchor are evaluated with respect to steric stabilization of liposomes. The branched polyether lipids possess a hydrophobic bis(hexadecyl)glycerol membrane anchor for the liposomal membrane. hbPG was chosen as a multifunctional alternative to PEG, enabling the eventual linkage of multiple targeting vectors. Overall, liposomes shielded by the branched polyglycerol lipids show a favorable biodistribution with greatly prolonged blood circulation times, rendering them promising novel nanovesicles for drug transport and targeting. |